The field experiments were conducted in a vape shop that was randomly selected (refer to the Methods section for details). This vape shop occupies a single room with an employee-only area on one side of the room. Approximately two meters away from where customers and employees vaped (Fig. 1b), the c-Air device was placed on the display countertop to capture e-cig generated aerosols. In addition to the c-Air device used within the vape shop, an Aerodynamic Particle Sizer (APS) was configured to take samples every two minutes to provide an independent measurement of the particle size distribution within the vape shop. The measured mean particle diameters are reported in Supplementary Fig. S1. Note that the particle sizing resolution of the APS device is < 500 nm. Therefore, the relatively large variations of the mean particle diameter shown in Supplementary Fig. S1 reflect the complexity and rapid changes of the indoor environment dynamics.
During these field experiments, c-Air was controlled by our team to collect air samples, with additional, on-demand measurements done upon the observation of vaping activities. The time between two consecutive c-Air measurements ({Delta }T_{k}) was kept between 5 and 30 min (Fig. 1c) with an average of ~ 16 min. Measurements were also done when no vaping was observed to ensure that ({Delta }T_{k}) was smaller than 30 min. Occasionally, multiple customer vaping events (e.g., continuous e-cig puffing in short periods of time) occurred between two consecutive c-Air measurements and these time instances were marked for further analysis. During each measurement step, c-Air sampled the indoor air for 60 s (sampling ~ 13L of air) and simultaneously recorded time-lapse inline holograms of the collected particles at 2 fps. After the initial 60-s time window, the vacuum pump stopped and the hologram recording continued for an additional 30 s, totaling 90 s of imaging time at 2 fps. A deep neural network20,25 was designed to reconstruct the captured time-lapse holograms (Fig. 2a), providing 180 complex-valued reconstructed images, each with independent amplitude and phase channels. At each time frame, the individual particles were detected via thresholding of both the amplitude and phase channels with an adaptive threshold of five standard deviations above the mean value of each image. The union of these binary spatial masks from both the amplitude and the phase channels provides an approximate boundary for each detected particle’s region-of-interest (ROI) at each time point. Having detected a collection of ROIs for all the captured aerosols, the particle volume at any time point t (see e.g., Supplementary Videos 1–3) was estimated by integrating the phase values ((phi)) within each one of these ROIs, i.e.,
$$Vleft( t right) = frac{lambda }{{2pi {Delta }n}} cdot mathop sum limits_{i,j} phi left( {Ileft( {i,j;t} right)} right)$$
(1)
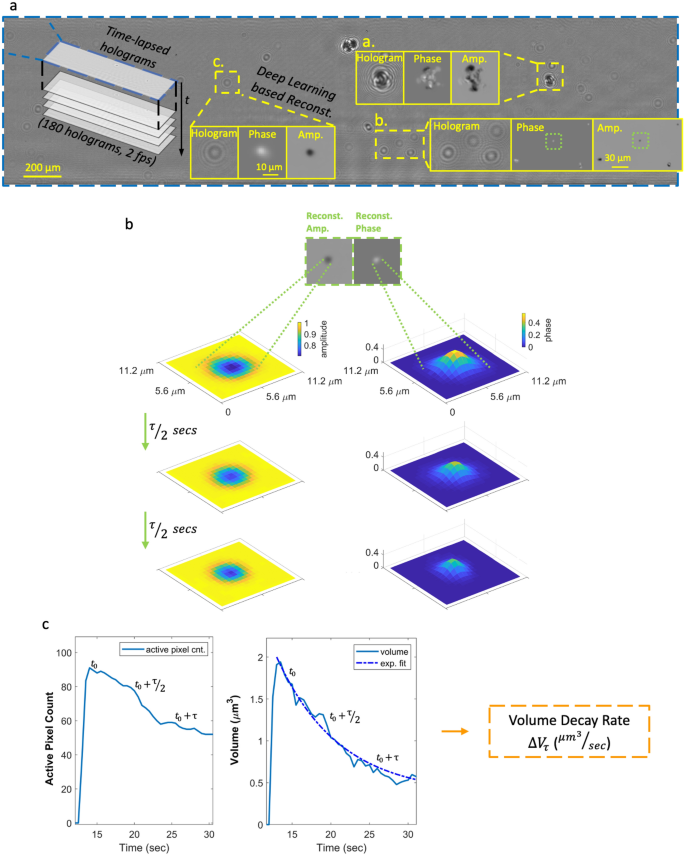
Calculation of the volatility of each detected aerosol: from raw holograms to volume decay rate. (a) A full field-of-view raw inline hologram and some example reconstructions showing the phase and amplitude channels separately. (b) An example aerosol image and the evolution of its holographic phase and amplitude channels at time points 0, τ/2 and τ, where τ is the exponential time constant (see Eq. (3)). (c) The plot of the active pixels in the amplitude channel as a function of time (left) and the exponential decay of the particle volume (right).
where we assumed a refractive index difference of ({Delta }n) = 0.4 with respect to air/vacuum; this is a reasonable assumption as it reflects the typical refractive index value for PG and VG32,33. The phase values were unwrapped and the majority of the particles had phase values between [0, 2 (pi)]. In this equation, (lambda) is the illumination wavelength (850 nm) and I is the reconstructed complex image of each particle within the ROI. The summation in Eq. (1) is carried over a set of lateral pixels {i,j} defined by an additional spatial mask generated specifically for the particles of interest. These binary masks were created by taking the pixels that are at least one standard deviation above the mean value of all the pixels in the ROI.
Based on Eq. (1), the volume decay of each aerosol was approximated using an exponential fit:
$$begin{array}{*{20}c} {Vleft( t right) = alpha e^{ – kappa t} + b} \ end{array}$$
(2)
In Eq. (2), the exponential term was optimized to fit the volume decay of the particle, whereas the second term was optimized to match any time-invariant term, representing some of the particles that do not fully evaporate (see e.g., Supplementary Videos 2 and 3). The lifetime of the particle, (tau), is further defined as:
$$begin{array}{*{20}c} {tau = frac{1}{kappa }} \ end{array}$$
(3)
To a first-order approximation, the retrieved κ value does not depend on the initial particle size and volume, and therefore can be considered a figure of merit for the volatility of an evaporating particle.
To be able to quantify particles with different sizes, the initial volume of a particle, (V(t_{0} {)}), right after it lands on the collection substrate of c-Air is divided by its corresponding lifetime (tau):
$$begin{array}{*{20}c} {Delta V_{tau } = frac{{V(t_{0} {)}}}{tau }} \ end{array}$$
(4)
giving us the volume decay rate (Delta V_{tau }) (µm3/sec) (see Fig. 2b, c).
During the four days of c-Air experiments in the vape shop, we conducted a total of 115 different measurements, whereas the APS sampled the air at a much faster rate of one sample per two minutes. The particle emission due to human vaping is depicted by the sharp rises in particle concentration measured by APS (see the red bars in Fig. 3). To reveal the dynamic changes of all the collected particles (usually on the order of hundreds to thousands of particles per c-Air measurement), the average volume decay rate of each measurement was calculated and plotted in Fig. 3, blue dotted lines. Fourteen of these measurements were taken when there was no vaping observed, and these pre-vaping points were marked using solid dots in the plots. The volume decay rate (μm3/sec) quantifies a particle’s evaporation speed, and therefore higher volume decay rates indicate faster evaporation and higher volatility. The correlation that is observed in Fig. 3 between the dynamics of the mean volume decay rate and the APS measurements reports a link between the e-cig emissions of vapers and aerosol volatility (Fig. 3). Also, note that the majority of local minimums and maximums of the c-Air measured volume decay rates reported in Fig. 3 for different measurement days match the corresponding local extreme points in our APS measurements. The mismatch between APS measurements and our c-Air analysis results might be due to the nonhomogeneous air circulation within the room, since these devices were placed at different spatial locations.
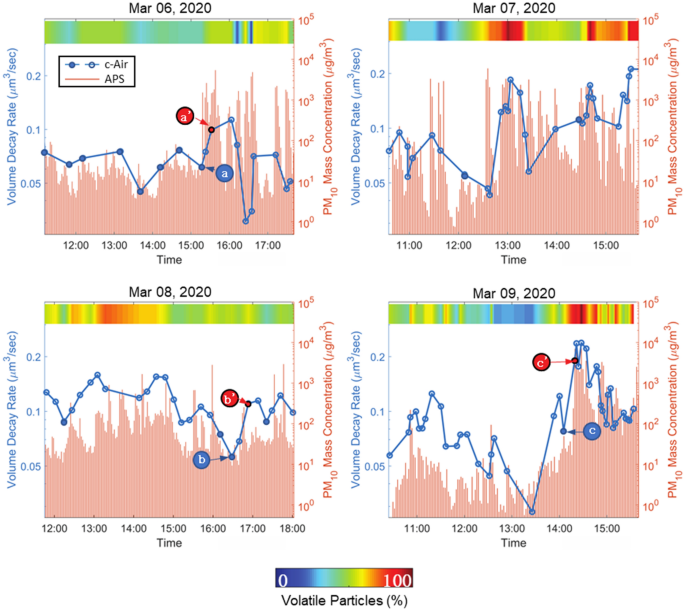
The change of the mean volume decay rate and the percentage of volatile aerosols throughout different days of experiments. Solid points refer to the pre-vaping measurements. (a), (b) and (c) mark the data points where there was no observation of a vaping event prior to c-Air sampling, whereas, a’, b’ and c’ mark the measurement points where vaping was observed in the vape shop.
Given the complex chemical composition of the captured e-cig aerosols (from various e-liquids), we defined three categories of aerosols according to the volumetric evolution of the imaged particles: volatile, semi-volatile and non-volatile particles (see Fig. 4 and Supplementary Videos 1–4). A particle is defined to be volatile if it fully evaporates (i.e., its volume gets smaller than an empirical threshold of 0.1 μm3 before the end of each measurement) and its volume exhibits a smooth exponential decay (see e.g., Supplementary Video 1). Since the c-Air device records an additional set of 60 frames after the air sampling is complete, all the volatile particles that were captured by c-Air fully evaporated within our observation time window. The mean and standard deviation of the volume decay rates of all the volatile particles captured in 115 measurements were used to further differentiate the remaining particles, forming an empirical definition for semi-volatile particles, i.e., they exhibit an initial volume decay, followed by a second phase where they remain larger than 0.1 μm3 in volume. Semi-volatile particles can be coagulated particles or particles with solid cores10,34 (see e.g., Supplementary Videos 2 and 3). The remaining particles that did not exhibit detectable evaporation were defined as non-volatile particles. These particles exhibited a very small volume decay rate which was empirically found to be less than 0.05 μm3/sec. For these non-volatile particles, the subtle changes in their measured volumes as a function of time might be related to the deformation of the sampling pad after the particle’s impaction (see Supplementary Video 4). Also, the changes in moisture, especially with humectants like PG and VG, might also result in subtle differences in the measured volumes. For each c-Air measurement point, the percentage of volatile and semi-volatile particles combined is color-coded and shown in Fig. 3. The change in the ratio of the volatile particles to other detected particles was observed to be similar to the time dynamics of the volume decay rate that we measured using c-Air (see the colorbar in Fig. 3 for each measurement day). This indicates that the percentage of the volatile particles within the detected aerosols can be used as an indirect measure of aerosol volatility and volume decay rate.
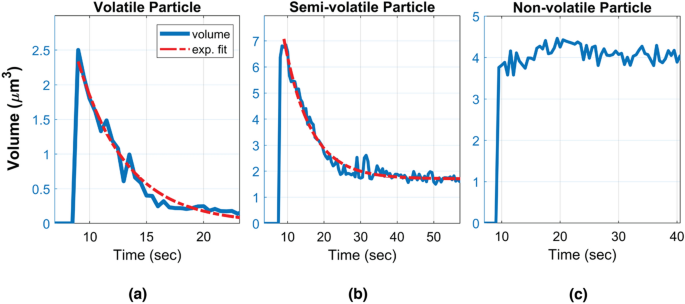
Characteristic volume changes of a (a) volatile, (b) semi-volatile and (c) non-volatile particle, sampled within the vape shop.
To further expand our analysis, we compared the statistical distribution of the volume decay rate of the particles captured post-vaping and pre-vaping by applying a Wilcoxon rank-sum test35, as shown in Supplementary Fig. S2, which tests the null hypothesis that the volume decay rates are sampled from continuous distributions with equal medians. A statistically significant increase in the volume decay rate of post-vaping aerosol measurements was observed with p < 0.05. As part of this analysis, we focused on three pre-vaping measurements, i.e., a, b and c marked in Fig. 3, that were conducted while no human vaping was observed in the vape shop. Given the dynamic indoor environmental conditions throughout a day, in terms of e.g., temperature, humidity and opening/closing of the outside door, these vape-free measurements within the vape shop can be considered as a background state for comparison purposes. An increase in aerosol volatility and volatile particle ratio can be clearly seen in the following three subsequent measurements points a’, b’ and c’ (see Fig. 3) during which the customers in the shop vaped, significantly increasing the e-cig generated aerosols. Note that these time spots were not the only vaping events observed in our field testing. Furthermore, dense and rapid vaping events within the vape shop also increased the aerosol volatility that we measured. For example, a continuous burst of vaping was observed close to our c-Air device on March 9th, at around 14:30 PM (Fig. 3). As a result of this continuous vaping within the shop, a sharp rise in PM10 emission, particle volatility, as well as volatile particle ratio, is clearly observed in Fig. 3. In this case, c-Air measurements revealed that > 95% of the particles fell in the category of volatile particles and the measured volume decay rates were also notably higher compared to other measurement points within the same day, which shows how dense, successive vaping events can significantly increase the aerosol volatility within a room.
Several limitations of the presented research might affect its generalizability. First, the chemical composition of secondhand e-cig aerosols remained unknown during the field testing (involving unknown, random customers/vapors), and the environmental variables such as the room temperature and humidity were not measured/controlled. Second, the devices we used (APS and c-Air) were placed at different spatial locations. Without sufficient ventilation, the measurement results of the two devices might not be exactly comparable. Third, the sampling using c-Air device was manually controlled, providing variable time intervals between vapes and measurements. Finally, the interactions between the droplets and the sampling substrate remained uncontrolled; liquid diffusion inside the sampling pad might generate some uncertainty on our estimation of volatility. Further improvements of the c-Air device and more controlled experiments (without impacting the cost-effectiveness and the field-portability of the measurement system) can be used to provide more insightful information regarding indoor vaping.
In conclusion, we conducted field experiments in a vape shop to characterize the volatility of e-cig emission using a field-portable, high-throughput device which can sample aerosols at a rate of 13 L/min and continuously image the collected particles on an impaction-based sampler. These field experiments were carried out in a vape shop where the customers and employees vaped their own e-cigs, resulting in the vaporization of e-liquids with different chemical constituents. The c-Air device enabled us to image these microscale particles generated by e-cigs during their evaporation lifetimes, allowing us to quantify their numbers, size and volatility. These field experiments revealed that vaping in the vape shop resulted in an increase in the percentage of volatile and semi-volatile aerosols during the successive post exhalation time.
https://www.nature.com/articles/s41598-022-07150-2